
How Are Polyhydroxyalkanoates (PHAs) Produced?
Polyhydroxyalkanoates (PHAs) are aliphatic polyesters that can be synthesized by bacteria, archaebacteria, cyanobacteria, algae, fungi, and other microorganisms (Taguchi et al., 2003; Behera et al., 2022). This blog will dive into some of the main methods of PHA production, their benefits and constraints, and some of the proposed solutions that could lead to more sustainable and cost-effective production of PHAs.
Microbial Fermentation for PHA Production
Microbial fermentation involves a process whereby microorganisms, such as bacteria, archaebacteria, and fungi, break down large organic molecules (e.g., carbohydrates) into simpler ones (Sharma et al., 2020). Polyhydroxyalkanoates (PHAs) are synthesized via fermentation of organic compounds (e.g., glucose) and other carbon substrates inside a microorganism, which accumulate PHAs intracellularly as carbon and energy storage materials (Bhuwal et al., 2013; Behera et al., 2022; Acharjee et al., 2023).
Different microorganisms and cultivation conditions can yield PHA homo- or copolyesters of 3-, 4-, 5-, and 6- hydroxyalkanoic acids. Poly(3-hydroxybutyrate) [P(3HB)] polymers, a well-researched subclass of PHAs, were the first to be discovered and commercialized (Tan, 2017). The first phb gene was isolated from Zoogloea ramigera, which is an aerobic bacteria used to engineer biopolymers that produces both P(3HB) and extracellular polysaccharide. Since this discovery, many other genes that encode enzymes from PHA biosynthetic pathways have been cloned from different microorganisms (Madison & Huisman, 1999).
There are also over 90 currently known genera of both Gram-positive and Gram-negative bacteria that can synthesize PHAs in both aerobic and anaerobic conditions (Raza et al., 2018). According to Yadav et al. (2021), there are over 300 bacteria, archaebacteria, and other microbial species known to date that have the capability to produce PHAs.
Bacteria can be categorized into two main groups when it comes to PHA production:
- Bacteria that accumulate PHAs when nutrients (e.g., nitrogen, phosphorus, oxygen) are limited, but this accumulation does not occur during the growth phase in the cultivation medium (Akaraonye et al., 2010; Raza et al., 2018).
- Bacteria that have no nutrient limitation requirements yet synthesize and accumulate PHAs during the growth phase in the cultivation medium (Akaraonye et al., 2010; Raza et al., 2018).
Carbon sources
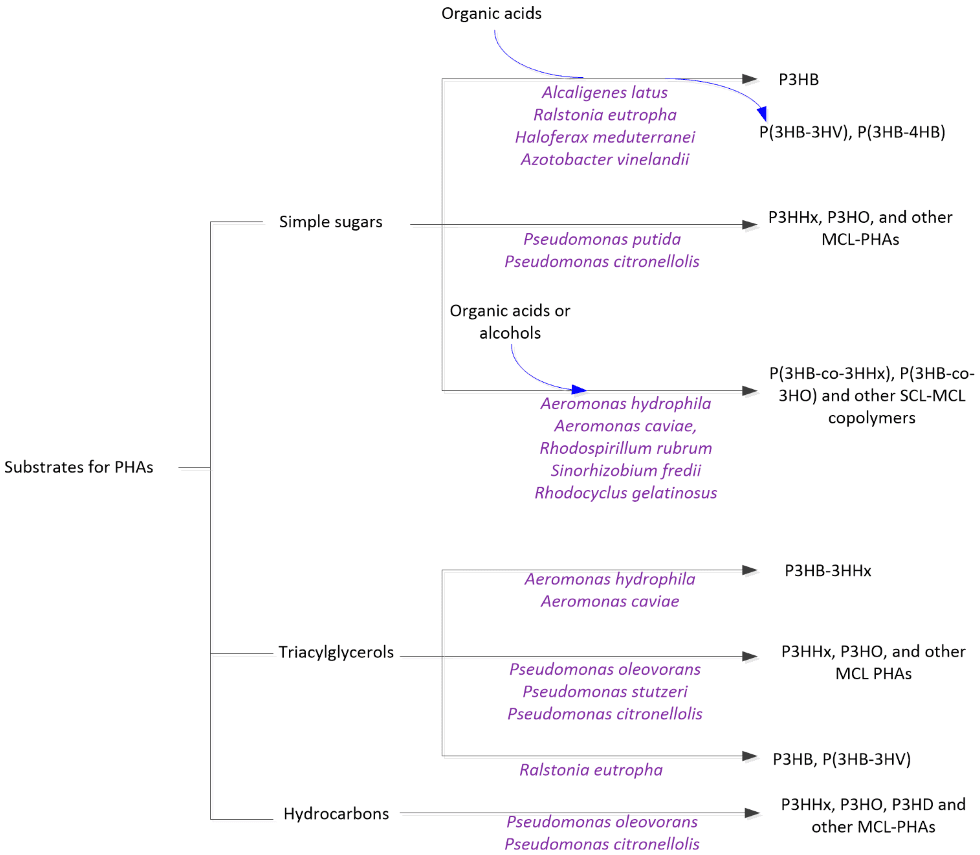
Key to PHA synthesis in bacteria is the carbon source, as it affects the composition of PHAs produced by different bacterial strains (Raza et al., 2018). These sources are broadly categorized as starch-based, sugar-based, cellulosic, hemi-cellulosic, whey-based, and oil- and glycerol-based media. As shown in Figure 1, these can be further simplified into three general substrate categories: carbohydrates, hydrocarbons, and triacylglycerols Jiang et al., 2016).
Bacteria convert the substrate/carbon source into PHA biopolymers through a series of enzymatic reactions. To trigger PHA production, the nutrient supply must be carefully managed. When bacterial growth is limited by depletion of nutrients, such as nitrogen or phosphorus, bacteria are naturally prompted to store carbon and energy (Verlinden et al., 2007; Shang et al., 2003). Nitrogen limitation is a common strategy to redirect many microorganisms’ metabolic pathways toward PHA synthesis, while for bacteria such as Azotobacter spp., limiting oxygen is more effective for synthesis (Amache et al., 2013; Verlinden et al., 2007).
Fermentation conditions
The physical properties and degradation rate of PHAs can be modified by changing the bacterial source and the fermentation conditions (Akaraonye et al., 2010). These fermentation processes for microbial production of PHAs take place in controlled environments, where factors such as temperature, pH, oxygen levels, and agitation are optimized for microbial growth and PHA accumulation. Three main bacterial cultivation strategies include (Vigneswari et al., 2021; Amache et al., 2013):
- Batch fermentation: this common method is known for its simplicity, flexibility, and low operation costs. The substrate and other requirements are added to the bioreactor at the beginning, allowed to react with one another, and then after the reaction the PHA material can be extracted. However, studies have shown that polymer composition is not constant over time (especially as compared to continuous/chemostat cultures) (Amstutz et al., 2019).
- Fed-batch fermentation: also known as semi-batch fermentation, this favored strategy is considered the most efficient for achieving high cell density cultivation, high yield, and high performance. During operation, this process involves adjusting culture broth feed rates by adding nutrients needed for cell growth or product formation in the culture vessel—either intermittently or continuously—to keep the concentration of limiting nutrients at an optimal level. Productivity is increased while overall fermentation time is decreased (Poontawee & Limtong, 2020).
- Continuous fermentation: also known as chemostat fermentation, this method involves continuously replacing culture broth with sterile medium. This steady-state process requires that the microbial culture be continuously fed nutrients at a fixed rate, while simultaneously being harvested to keep culture volume constant. Since physiological parameters of cultures in stead-state do not change over time (theoretically), growth conditions can be studied in a time independent manner (Amstutz et al., 2019).
Extraction of PHAs
After fermentation, PHA needs to be extracted from the bacterial cells and purified. Table 1 reflects the typical steps for downstream processing associated with bacterial synthesis of PHAs, along with examples of methods for each step:
| Biomass Separation | Pre-Treatment | PHA Recovery | PHA Separation | PHA Purification |
|---|---|---|---|---|
|
|
|
|
|
Table 1 : Steps in PHA downstream processing and example methods for each step (adapted from del Oso et al., 2021).
The below list provides more detail on some of these downstream processing methods, specifically with regard to extraction/recovery of PHAs (Raza et al., 2018):
- Solvent extraction: this is considered the most common method for PHA extraction. First, bacterial cells are ruptured through a pre-treatment process that exposes PHA granules. The granules are then solubilised in chloroform or other chlorinated solvents, and finally precipitated with ethanol or methanol. This method does not degrade the polymer, removes cell endotoxins, and creates a high purity PHA product. However, halogenated solvents like chloroform require a lot of energy input (which increases production costs) and are harmful to the environment (Rodrigues et al., 2022).
- Floatation method: this process first uses a solvent such as chloroform to extract PHAs from bacterial cells, with an added step of leaving this cell-chloroform mixture for a period of time so that floatation of cell debris can occur. Raza et al. mention that this method can employ the use of environmentally friendly solvents, and that the process is known to reduce wastage of the extracted polymer (Raza et al., 2018).
- Digestion method: as an alternative to solvent extraction, this method releases PHAs from bacterial cells via chemical or enzymatic digestion. Chemical digestion involves sodium hypochlorite or surfactants to recover PHAs from cells, while the enzymatic method involves heat treatment, enzymatic hydrolysis, and surfactant washing. However, chemical digestion can be problematic if toxic substances such as chloroform are used (Raza et al., 2018).
- Supercritical fluid extraction: by raising temperature and pressure of a substance beyond its critical point, one can create highly compressed fluids that combine the properties of both gases and liquids. Supercritical carbon dioxide, ammonia, and methanol extraction has been used to recover PHAs from bacterial cells. This method is effective at extracting endotoxins and other impurities that may remain after solvent extraction, which is very important for producing PHAs used in vivo for biomedical applications (Raza et al., 2018).
- Aqueous two-phase extraction (ATPE): as a non-solvent method, ATPE is considered an ecologically friendly approach. It uses water to isolate, purify, and recover PHAs from bacterial cells (Raza et al., 2018).
Though final PHA production costs depend on a variety of factors, downstream processing is said to be responsible for 50% or more of the total cost of production (Saratale et al., 2021; Vu et al., 2021).
Constraints of microbial fermentation for PHA production
Microbial fermentation is currently a main industrial biotechnology method for producing polymers such as PHA. This method of production is associated with high energy consumption and maintenance costs for tasks such as sterilization, oxygen supply, and agitation—all of which contribute to high overall PHA production costs (Dong et al., 2023). Microbial fermentation methods also face obstacles (Chen & Jiang, 2018; Rodrigues et al., 2022; Chen et al., 2020) related to:
- Frequent microbial contamination (and high capital investment for facilities/equipment needed to mitigate contamination),
- High energy and water resource needs,
- Difficulty with recycling culture broth,
- Complex and costly product separation and purification (e.g., downstream processing),
- Low substrate to product conversion efficiency, and
- Lack of platform microbial strains to develop multiple products in shorter time periods.
Other considerations regarding microbial fermentation for PHA production include:
- Fermentation/cultivation strategies: each of the three main fermentation strategies has its benefits, as well as drawbacks.
- Batch fermentation has the lowest productivity out of the group, since the accumulated PHA begins to deteriorate after the carbon source is fully expended, resulting in an overall decrease in PHA material.
- The overall PHA production of fed-batch cultivation is considered low when nitrogen is the limited nutrient; combining batch and fed-batch processes has become a common fermentation strategy for higher productivity.
- Though chemostat/continuous fermentation is known for high controllability, the continuous feeding process for PHA build-up, yield, and productivity is also associated with a higher chance for contamination (Vigneswari et al., 2021).
- Bacterial strains: efficiency of PHA production varies among different strains. The development of genetically engineered strains with higher PHA accumulation rates and broader substrate utilization is one of the methods being explored to increase efficiency and yields (Kourmentza et al., 2017).
- Scaling: scaling up PHA production from lab-scale to industrial-scale can be complex. In addition to high costs, challenges such as maintaining optimal conditions, preventing contamination, and achieving consistent PHA quality are among the list of hurdles.
- Sustainability of carbon sources: sustainability of carbon sources used in PHA production is critical. Researchers are exploring alternative feedstocks, such as lignocellulosic biomass and agricultural waste, to reduce the reliance on food-based substrates that require large amounts of land, water, and other resources to maintain (e.g., Koller et al., 2005; Vigneswari et al., 2021).
Despite these challenges, research and innovation hold the promise for efficient methods for PHA production via microbial fermentation. For example, microbial fermentation methods for PHA production began with the use of pure bacterial cultures, with a pure concentrated substrate (e.g., glucose). However, PHAs can also be produced in open mixed-culture systems using diverse microbial communities and a broader range of substrates, including activated sludge from wastewater treatment plants (Rodrigues et al., 2022). These systems allow for the use of a wider variety of cheaper carbon sources and continue to pique interest due to their ability to produce higher PHA yields and lower overall production costs (Salehizadeh & Van Loosdrecht, 2004; Gurieff and Lant 2007; Kourmentza et al., 2017).
Microalgal Biomass for PHA Production
Microalgae, such as cyanobacteria and eukaryotic algae, have garnered attention as excellent biomass candidates for PHA production due to their rapid growth rates, high photosynthetic efficiency, and ability to accumulate PHAs intracellularly. Microalgae have fewer nutrient requirements for growth as compared to typical bacterial PHA synthesis, and they are the only microorganisms that accumulate PHAs via photosynthesis (Costa et al., 2019).

Algae-based PHA production involves several key steps (Chia et al., 2020; Chong et al., 2022):
- Strain selection: identifying and selecting algae strains with high PHA-producing capabilities is the first crucial step. Researchers often genetically modify these strains to enhance their PHA production potential.
- Cultivation: microalgae are typically cultivated in closed photobioreactor systems under controlled conditions. However, large-scale cyanobacterial culture occurs in outdoor open pond systems that are dependent on natural light.
- PHA accumulation: algae accumulate PHAs inside their cells in response to nutrient limitations. This natural process can be optimized by manipulating environmental conditions.
- Harvesting: once the algae have accumulated sufficient PHAs, they are harvested from the culture and separated from the growth medium.
- PHA extraction: PHAs are then extracted from the harvested biomass and purified for further processing.
Benefits of using microalgal biomass for PHA production
Algae-based PHA production is versatile, as algae can be cultivated in various environments, such as wastewater treatment plants, saltwater environments, and freshwater bodies. Through photosynthesis, algae consume CO2 as a main source of energy, which means that algae-based PHA production reduces both fossil fuel use and carbon dioxide emissions—making this an environmentally appealing method with a notably lower carbon footprint (Costa et al., 2019).
This method of production also does not need to compete with food crops (and the land, water, and nutrients needed), since microalgae can be grown on waste resources with high lipid accumulation (Chia et al., 2020). Microalgal biomass can also grow very quickly, can survive high temperatures and other harsh conditions, has high carbon fixing efficiency, and is a promising feedstock for creating sustainable third-generation biofuels (Arora et al., 2023). Widely-consumed first-generation biofuels come from food crops (e.g., sucrose- and starch-derived bioethanol), and non-food lignocellulosic bioethanol crops are an example of second-generation biofuels that were created to mitigate competition with food crops (Chen et al., 2015).
Researchers have advocated for integrated microalgae biorefineries for a circular economy, since microalgae can not only produce third-generation biofuels, but also high value co-products that could help offset the high capital investments and operations needed for a biorefinery alone (Chew et al., 2017). High value products include proteins, carbohydrates, and lipids that can be produced in large amounts in relatively short time periods, as well as PHAs and other bioplastics, pigments, and vitamins and antioxidants (Chong et al., 2022; Chew et al., 2017; Arora et al., 2023). Microalgal biomass also has potential as filler for enhancing bioplastic properties.
Constraints of using microalgal biomass for PHA production
As is the case with other methods, algae-based PHA production has its own hurdles to overcome. It is important to choose the right microalgae to determine biomass productivity and define the end products of downstream PHA synthesis (Tan et al., 2022). Wild type microalgae that provide high biomass productivity include Arthrospira (formerly known as Spirulina), Chlorella, and Chlamydomonas reinhardtii; however, these and other microalgae strains typically have yields that are much lower than those acquired via microbial fermentation methods.
Bioengineering of microalgal species can help to overcome this issue, though this is still considered a nascent field of study. Methods used for gene sequence modification of microalgae include Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and Transcription Activator-Like Effector Nucleases (TALENs). To activate or repress particular microalgae genes, techniques include short interfering RNAs (siRNA) and microRNAs (RNAi) (Tan et al., 2022). Each of these methods also have their advantages and disadvantages, which Tan et al. discuss in more detail.
While algae can be genetically engineered to create more productive strains, factors related to horizontal gene transfer, outcompeting wild-type strains, regulatory hurdles (e.g., GMO certifications), modified organisms spilling into and having adverse effects on natural ecosystems are among some of the concerns associated with genetic modification of microalgae strains (Chia et al., 2020).
Cultivation methods also come with some limitations. Open pond cultivation has low operating costs and is relatively easy to maintain and scale. However, it is difficult to cultivate certain algal strains on a large scale, and there are issues related to lower productivity and higher contamination risk. Closed photobioreactor systems have higher (and more diverse) strain yields and lower contamination risk, but have much higher scale-up costs as compared to open systems (Chia et al., 2020; Arora et al., 2023).
Despite these challenges, researchers continue to look for more efficient and economical ways to produce PHAs from algal biomass. For example, successful and economical PHB production has occurred when recombinant E. coli is grown on hydrolyzed wastewater algal biomass (Arora et al., 2023). However, PHA/PHB is often accumulated inside microalgae cells at low percentages by weight, which has further reinforced calls for research on gene-editing tools like CRISPR to modify enzyme producing PHB/PHA content in microalgal species as a way to scale the production process (Arora et al., 2023).
Next Generation Industrial Biotechnology for PHA Production
This blog has covered a number of different challenges associated with synthesizing PHAs in vivo using microbial chassis. These methods are among the current industrial biotechnology (CIB) approaches that produce bio-products, including bulk chemicals, PHA and other polymeric materials, and biofuels that rely on the bioprocessing of starch, fatty acids, and other agriculture products (Chen & Jiang, 2018).

Next Generation Industrial Biotechnology (NGIB) is an energy- and resource-saving approach that relies on extremophilic bacteria grown on low-cost mixed substrates to make the production of bio-products, including PHAs, more cost-effective and competitive (Chen & Jiang, 2018; Yu et al., 2019; Chen et al., 2020; Ye & Chen, 2021). NGIB is meant to be a simplified approach that relies on contamination-resistant microorganisms, such as the Gram-negative halophilic (salt-tolerant) aerobic bacteria of the Halomonas spp., that can be inoculated by growing in environments of high salinity and alkalinity (Chen & Jiang, 2018; Ye & Chen, 2021). It also incorporates bi-directional agitation bioreactors and advanced metabolic engineering techniques.
Table 2 provides a brief, non-exhaustive overview of how NGIB aims to avoid and/or overcome the challenges associated with CIB.
| Current industrial biotechnology (CIB) | Next Generation Industrial Biotechnology (NGIB) | Process(es) needed to achieve NGIB |
| High freshwater consumption | Low freshwater dependency | Seawater-based media, recycling process water |
| High energy consumption | Reduced energy consumption | Open, unsterile, and continuous processes |
| Low conversion of substrate to product | Convert more substrate to product | Remove and/or weaken competing pathways |
| Microbial contamination | Contamination-resistant microbes | Screen for robust microbes |
| Limited conditions for microbial growth | Flexible growth conditions | Selecting robust microbes |
| Batch fermentation processes | Continuous processes | Contamination-resistant microbes |
| Extended time periods for cell growth | Accelerating cell growth | Using synthetic biology to speed up growth |
Table 2: Comparing current industrial biotechnology (CIB) with Next Generation Industrial Biotechnology (NGIB). Adapted from “Next generation industrial biotechnology based on extremophilic bacteria” by Chen & Jiang, 2018.
The approaches listed in Table 2 and further detailed by Chen & Jiang are meant to increase the cost effectiveness and decrease the process complexities of PHA production and other bioprocesses. Research on how Halomonas spp. and other extremophiles and halophiles can support the commercialization of PHAs and other bio-products is ongoing, and future discoveries are likely to transform the capabilities of NGIB approaches.
Cell-Free Methods for PHA Production
Current microbial bioprocessing is influenced by the inherent limitations of cells, such as the need to balance intracellular fluxes for managing active synthetic pathways, while still maintaining the growth and maintenance needs of the host (Dudley et al., 2015). Cell-free production methods are not bound by the same limitations, offering an approach that holds immense potential for more economical and efficient PHA production.
These in vitro methods leverage cell-free systems (CFSs), such as cell lysates (i.e., materials produced by breaking down cell membranes) or purified enzymes to directly synthesize PHAs from simple carbon sources (Yang et al., 2023). CFSs contain the cellular components that are needed for transcription and translation (Choi et al., 2023). Research on in vitro PHA synthesis using purified enzymes began in the 1970s and has since been widely adopted and supported by a growing body of research (Dudley et al., 2015; Dong et al., 2023).
Benefits of cell-free PHA production
CFSs have several advantages for PHA production. PHA synthesis is disconnected from the generation of bacterial biomass, allowing in vitro CFSs the potential to overcome some of the issues associated with microbial cell-based PHA synthesis (Dong et al., 2023).
By eliminating the need for living cells, cell-free production offers numerous advantages, including precise control over metabolic pathways, faster production rates, and reduced bioprocess complexity. Moreover, it enables the utilization of diverse feedstocks, which can contribute to more sustainable PHA production. Dong et al. provide an in-depth discussion on the opportunities associated with cell-free PHA production. Below are a few of their highlights to summarize:
- High potential to accelerate the design-build-test (DBT), as cell-free methods can avoid cell growth and product synthesis resource conflicts (as cited in Grubb et al., 2020).
- Ability to complement in vivo metabolic engineering.
- Can offer several advantages that address “bottleneck” issues in PHA synthesis, such as:
- Eliminating cell volume limitations via easy separation of PHA from the CFS, which also enables longer, continuous productivity.
- Achieving real-time monitoring of product concentration (e.g., PHB content), in contrast to the issues associated with real-time detection of intracellular PHA content (which can require post-fermentation analysis).
- Reducing product separation costs that allows for more sustainable and cost-effective PHA production. In vitro synthesis only needs centrifugation to retrieve high purity PHA products, bypassing the need for current energy-intensive cell crushing and environmentally unsafe solvents (as cited in Hodgman & Jewett, 2012).
- Providing better control of monomer composition, as the CFS combines enzymes, polymerases, and specific substrates to produce “tailored biopolymers with specific and customizable properties, facilitating the study of PHA material properties and expanding their potential applications.”
Constraints of cell-free PHA production
As with all the production methods discussed so far, CFSs also have barriers that need to be overcome before the method can contribute to large-scale production of PHA bioplastics (Dong et al., 2023). A few of these are listed below:
- While the monomer composition can be relatively well controlled via in vivo PHA synthesis, in vitro synthesis may face insufficient diversity in monomer composition (Dong et al., 2023).
- One reason is that complex coenzymes and/or acyl-carrying proteins are needed to execute fatty acid synthesis and degradation pathways in vitro, particularly for medium- and long-chain length PHAs (mcl/lcl PHA).
- Progress for mcl-PHA in vitro synthesis remains limited, and there is an inhibitory effect that acetyl-CoA has on PhaC (a PHA synthase) in the PhaCAB pathway (that produces poly-3-hydroxybutyrate) that also need to be overcome (Hiroe et al., 2012).
- Activity and stability of enzymes in CFSs are often lower than those in live cells. Solutions to address this may include the use of thermostable enzymes, protein engineering, or multienzyme immobilization technology (Dubey & Tripathi, 2021 and Phan et al., 2022).
- The cost to regenerate co-factors, such as NAD(P)+, can be high. Potential solutions include the integration of coenzyme reactions and/or using less expensive substrate cofactors that could regenerate spent co-factors (Dong et al., 2023).
Despite the challenges associated with cell-free PHA production, it’s important to consider the novelty of this approach and the room that remains for additional research and innovation.
Conclusion
The methods outlined in this blog are by no means exhaustive. There are many active investigations into how to make polyhydroxyalkanoate production more sustainable that will no doubt influence an ever-growing body of research on this and related topics. For a deeper dive into some of the PHA production approaches mentioned here, Kourmentza et al. (2017) offers an in-depth review on the advances and challenges associated with PHA production, Tan et al. (2022) provides a thorough review of PHA production from microalgal biomass, Kelwick et al. (2018) discuss cell-free systems (including cell-free TX-TL systems), and Chen & Jiang (2018) (and other publications by Dr. Chen, including this interview) cover a myriad of current and future opportunities associated with Next Generation Industrial Biotechnology.
We are a team of molecular biologists, geneticists, and bioinformaticians brought together by the desire to solve difficult problems with far-reaching implications.
Address
© 2025 All Rights Reserved. Website by Astrael


