
What Are The Properties, Advantages, and Disadvantages of Polyhydroxyalkanoates (PHAs)?
Polyhydroxyalkanoates (PHAs) are a family of bioplastic materials. They are produced by microorganisms intracellularly as carbon and energy storage compounds (Behera et al., 2022). Microbes can be cultivated on a range of carbon sources, including sugars, lipids, and other organic materials (Jiang et al., 2016). In this blog, we’ll explore how PHAs are made, their basic properties, and some of their current advantages and disadvantages as a bioplastic material.
How Are Polyhydroxyalkanoates (PHAs) Made?
PHAs are made by microorganisms through the process known as polymerization. During this process, small molecules called monomers bond together to produce more complex chainlike structures called polymers (Helmenstine, 2020, Kjeldsen et al., 2018). Certain bacteria, archaea, and some fungi can accumulate PHAs intracellularly when they are provided with an excess of carbon sources while growth nutrients, such as nitrogen and phosphorus, are limited (Bhuwal et al., 2013, Acharjee et al., 2023). Enzymes facilitate the condensation of hydroxyalkanoic acid monomers, forming chainlike PHA polymers within the microbial cells.
PHAs are composed of various hydroxyalkanoic acids, which differ in the length of the alkyl side chain and the number and position of hydroxyl groups on the alkyl chain (See Figure 1). They are generally classified as either short-chain length PHAs (scl-PHA) of 3-5 carbon atoms, medium-chain length PHAs (mcl-PHA) with 6-14 carbon atoms, or long-chain lengths PHAs (lcl-PHA) with more than 14 carbon atoms (Muiruri et al., 2023; Kim, 2002).
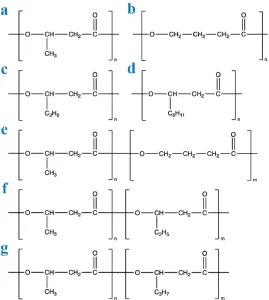
Figure 1: The chemical structures of polyhydroxyalkanoates (PHAs): (a) PHB. (b) P4HB. (c) PHV. (d) PHO. (e) P3HB4HB. (f) PHBV. (g) PHBHHx. Source: Guo et al., (2022). Polyhydroxyalkanoates in tissue repair and regeneration. Engineered Regeneration, 3(1), 24-40.
The monomeric unit composition significantly influences the physical and chemical properties of PHAs; this composition can vary depending on the microorganism and the feedstock source used for production (Amstutz et al., 2019; Philip et al., 2007). Common monomers in PHAs include 3-hydroxybutyrate (3HB), 3-hydroxyvalerate (3HV), 3-hydroxyhexanoate (3HHx), 3-hydroxybutyrate-co-3-hydroxyvalerate (3HBV), among many others (Song et al., 2022, Zhou et al., 2021, Choi et al., 2021).
The homopolymer of hydroxybutyrate poly(3-hydroxybutyrate) (PHB) was the first identified PHA bioplastic, discovered by French microbiologist Maurice Lemoigne in 1925. PHB polymers are a subclass of polyhydroxyalkanoates, and are the most researched of the PHAs (Kovalcik et al., 2019; Eppinger et al., 2011; Amstutz et al., 2019).
Properties and Advantages of Polyhydroxyalkanoates (PHAs)
Once PHAs are extracted from the cells of microbes, they can exhibit brittle thermoplastic and elastomeric properties, which can be managed by using different bacteria, fermentation, and substrate conditions (Philip et al., 2007; Rodriguez-Perez et al., 2018; García-Quiles et al., 2019).
PHAs also have the high melting temperature, high tensile strength, flexibility, and crystallinity properties of petroleum-based plastics (Akinmulewo & Nwinyi, 2019; Acharjee et al., 2023). In addition they are biocompatible and exhibit piezoelectricity, which makes them appealing for different biomedical applications (Behera et al., 2022).
PHAs are known for their ease of processing, high volume to surface ratio, and small pore size—factors that lend to their high capacity for recycling (Sharma et al., 2021). Other characteristics identified by Bugnicourt et al. (2014) include:
- Water insolubility and relative resistance to hydrolytic degradation (i.e., chemical breakdown when reacting with water),
- Solubility in chloroform and other chlorinated hydrocarbons,
- Resistance to UV light (but poor resistance to acids and bases)
- Ability to sink in aquatic environments, which helps facilitate anaerobic biodegradation in, for example, river sediments,
- Less ‘sticky’ qualities than traditional polymers when melted.
PHAs are both bio-based and biodegradable.
The “bio” in bio-based means that it is a material that is produced from a renewable biological source, also called a “feedstock”, such as vegetable oil, starch, and proteins (Behera et al., 2022). This is in contrast to traditional synthetic plastics that are derived from petrochemicals.
Biodegradable refers to the natural chemical process through which the material degrades via microbial action in specific environments under specific conditions (de Castro et al., 2022, Kjeldsen et al., 2018). PHAs can also be created from a variety of waste streams (e.g., paper mill effluents), which is a focus of research that aims to address issues with scaling PHA production in a more cost-efficient manner (e.g., Rodriguez-Perez et al., 2018; Abd El-malek et al., 2020).
Though a body of research mentions non-toxicity as another main characteristic of PHAs (e.g., Bugincourt et al., 2014; Pratt et al., 2019; Atiwesh et al., 2021; Jayarathna et al., 2022; Pandey et al., 2022; Ansari & Fatma, 2014; Acharjee et al., 2023 among others), concerns have emerged with regard to toxicity throughout the full life cycle of PHAs (e.g., Zimmerman et al., 2020). This is a topic that we examine more closely in our blog titled, “How Safe are Polyhydroxyalkanoates (PHAs) for the Environment?”
Biodegradability and Compostability
PHAs are considered to be the only 100% biodegradable polymers (Khanna & Srivastava, 2004). In accordance with American Society for Testing and Materials (ASTM) standards, PHA bioplastics can biodegrade in all anaerobic and aerobic environments (Acharjee et al., 2023).
With the right amount of oxygen and humidity, PHA bioplastic can degrade in aerobic conditions via microbial action into water (H2O), carbon dioxide (CO2), and biomass within 20-45 days, which is in stark contrast to the petrochemical plastics that persist in environments for hundreds or even thousands of years (Moshood et al., 2022). In anaerobic conditions, microorganisms degrade PHAs into CO2 and methane (CH4) in industrial facilities, soils, lakes, oceans, and even sewage (Khanna & Srivastava, 2004).
As explained by de Castro et al., (2022), biodegradation of PHA polymers in aerobic conditions occurs in the three stages of biodeterioration, biofragmentation, and assimilation (Figure 2):
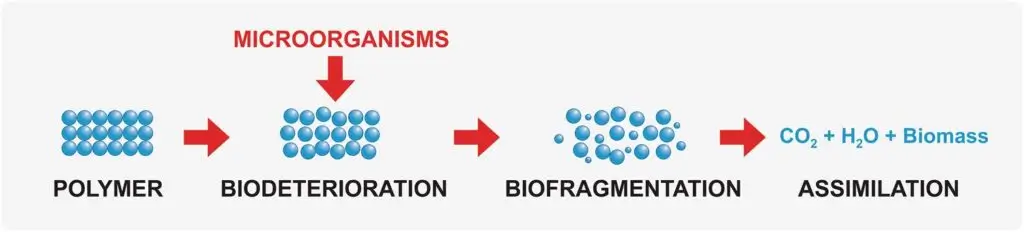
Figure 2: The Stages of polymer biodegradation. Source: de Castro et al., (2022). The Potential of Cleaner Fermentation Processes for Bioplastic Production: A Narrative Review of Polyhydroxyalkanoates (PHA) and Polylactic Acid (PLA).
Similar to PHA, other bioplastic materials can be both bio-based and biodegradable, though many tend to be one versus the other. For example, there are fossil-fuel derived bioplastics that can be biodegradable, but most are not. The terms “bio-based” and “biodegradable” are rarely defined on product labels and are often used interchangeably; however, these terms are not the same and this causes a lot of confusion among consumers and other stakeholders (Kjeldsen et al., 2018).
Figure 3 displays a bioplastics “material coordinate system” with four quadrants to illustrate these distinctions between material feedstocks and degradability: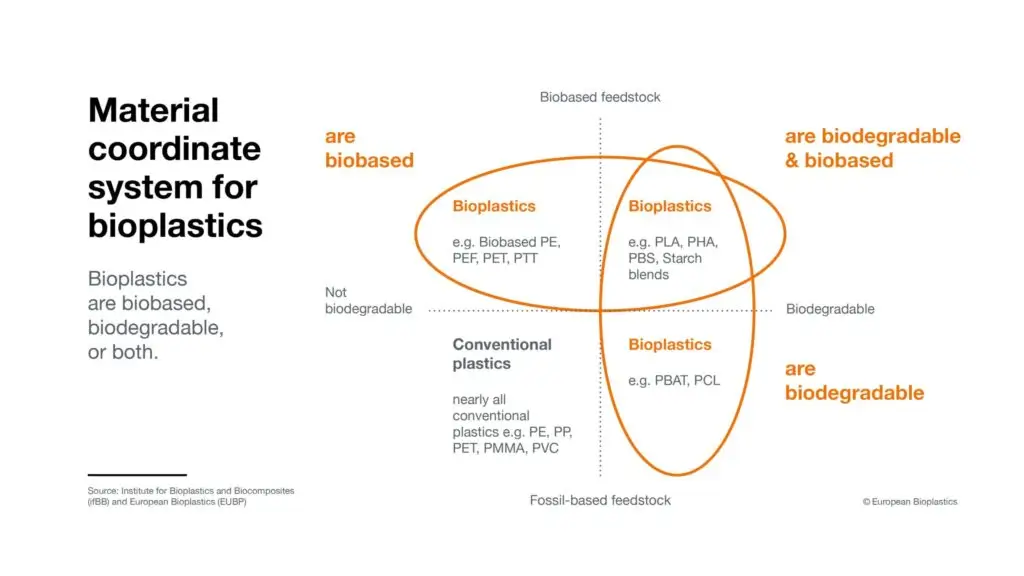
Figure 3, Quadrant 1 (top right) includes PHAs and other bio-based and biodegradable materials, such as:
- Poly(lactic) acid (PLA): this polymer is the most commonly used biodegradable polymer in medical applications and the second most traded polymer worldwide (da Silva et al., 2018). PLA has low heat resistance, which limits its applications; however, researchers (e.g., Zhao et al., 2022) continue to investigate ways to improve heat resistance by combining PLA with high heat-resistance polymers, fibers, or other materials so that biodegradable PLA could be used in a wider range of products.
- A main difference between PLAs and PHAs relates to compostability and biodegradability. DiGregorio (2009) cites the superiority of PHAs because they have been shown to meet the ASTM standard for biodegradability in marine environments. PLA, on the other hand, persists in marine environments for indefinite amounts of time. PLA is compostable in industrial environments, yet some reports indicate that PLA could be slightly toxic to compost microorganisms (Moore, 2008).
- A 2023 study by Royer et al. of Scripps Institution of Oceanography at UC San Diego revisited the biodegradability of PLA in marine environments and found that it did not degrade at all even after 428 days, and looked the same as the petroleum-based polypropylene and polyethylene terephthalate that was submerged with the PLA.
- Royer et al. (2023) highlighted that PLA is compostable under certain industrial conditions, but that this should not be considered synonymous with biodegradability in natural places such as the ocean. Rasal et al. (2010) also point to the slow degradation rate of PLA, and that it is a serious problem in relation to the disposal of consumer products and in some biomedical applications (e.g., PLA-based temporary implants that should, but don’t degrade).
- Polybutylene succinate (PBS): this is an aliphatic polyester that was traditionally produced from petrochemical sources, but over the last decade or so has been created from sugarcane, corn, cassava, and other renewable feedstocks. It is frequently blended with PLA, starch, proteins, and other polymers to improve material performance. It is used in many applications in the food & food packaging industry (e.g., coffee pods), in agriculture (e.g., mulch films), medical fields (e.g., drug encapsulation systems), and more. It is known to have good thermal stability and mechanical properties, and PBS-PHA blends can be used to further improve upon these properties (Aliotta et al., 2022; Rafiqah et al., 2021).
- Starch blends: starch can be used as a blend or composite with other polymers to make bioplastic materials (including blending with synthetic polymers to increase their biodegradability); however, starch is hydrophilic and lends to poor mechanical properties and water sensitivity of the final material (Jayarathna et al., 2022).
To be categorized as biodegradable, over 60-70% of the polymer product must degrade within six months (typically via a biodegradation test, either with compost or with activated sludge) (Hiraga et al., 2019). In Figure 1, Quadrant 2 (top left), we have bioplastic materials that are the same as PHAs in that they are bio-based, but different from PHAs because they are not biodegradable. Two examples include:
- Polyethylene terephthalate (PET) plastics : this is a thermoplastic polyester that is created by combining modified ethylene glycol and purified terephthalic acid. Bio-based PET is used for carbonated beverage bottles due to its heat and abrasion resistance, high strength, dimensional stability, and good chemical resistance. It is also used to make artificial textiles and fibers for sheets, upholstery, carpets, and more due to its excellent wear resistance, durability, and ability to not absorb a lot of moisture. PET is often labeled as “polyester” in these textile materials (Sin & Tueen, 2023).
- Polyethylene (PE) :bio-based PE is used in a wide variety of packaging applications and is being produced at a large scale, which is very different from PHAs that have a higher cost of production and are not yet at a large scale in the industrial market (European Bioplastics, 2022).
Figure 3, quadrant 3 (bottom left) lists conventional petroleum-derived plastics that are non-biodegradable, such as polyvinyl chloride (PVC), traditional PET and PE, and polypropylene (PP). Polyhydroxybutyrate (PHB), the first identified PHA, is comparable to conventional fossil-derived PP, with good resistance to moisture and excellent gas barrier properties—yet more appealing than PP due to its qualities as a bio-based, biodegradable, and non-toxic bioplastic material (Raza et al., 2018).
Quadrant 4 (bottom right) displays examples of conventional plastics that are biodegradable, such as poly (butylene adipate-co-terephthalate) (PBAT). PBAT is a petroleum-derived type of biodegradable aliphatic polyester that has good heat resistance and ductility, and is known for its processing performance, yet unlike PHA, it has poor crystallinity and low melt strength (Liu et al., 2022).
As discussed throughout this section, unlike these conventional plastics, PHAs are always derived from renewable organic resources (bio-based) and are additionally advantageous for their biodegradability in different industrial and natural environments.
Disadvantages of Polyhydroxyalkanoates (PHAs)
PHAs are a promising bioplastic, yet their widespread adoption still faces technological, economic, and environmental challenges. A brief overview is included in the sections that follow.
Costs
The high costs associated with the production of PHAs remains one of the biggest challenges to their industrial applications and widespread adoption. In contrast, the global production capacity of other bioplastics, such as polybutylene succinate (PBS), is much higher due to their significantly lower overall production costs as compared to PHAs (Meereboer et al., 2020).Downstream processing (i.e., difficulties with extraction and purification) is considered one of the biggest costs associated with PHA production (Rodrigues et al., 2022; Chen et al., 2020). Carbon sources metabolized by microorganisms and nutrients needed for balanced cultures can also contribute to higher costs of production, as do the costs of maintaining the fermentation process (Gomes Gradíssimo et al., 2020; Surendran et al., 2020). Another significant factor contributing to higher costs for PHAs is changing molecular weights (Mw) and structure related to varying PHA synthase activity during production, which affects its thermo-mechanical properties (Chen et al., 2020).
We’ll revisit these and other challenges associated with the high costs of PHA production, along with proposed solutions, in our blog titled, “How Are Polyhydroxyalkanoates (PHAs) Produced?”
Technical Challenges
During PHA production, lipid oxidation occurs and causes PHAs to incur “rancid” flavors and objectionable odors. Lipid residues and endotoxins can remain attached to the biopolymer after extraction, and these conditions are linked to the production of free radicals by autoxidation (García-Quiles et al., 2019). These unfavorable smells are considered an obstacle to broadening the industrial applications for PHAs, yet researchers are looking for ways to fix the issue. For example, García-Quiles et al. propose the use of nanoclays with high absorbance properties to capture the volatile compounds responsible for the unpleasant fragrance in PHAs.
Kovalcik et al. (2019) highlight that PHB, currently the most popular PHA on the market, has a very narrow thermal processing window. This causes thermal degradation issues such as the quick and substantial decreases of molecular weight, changes in color from white or yellow to brown, and the loss of final rheological and mechanical properties.
Resource Considerations
A main concern associated with PHA production is the use of substrates that are primarily derived from food-based carbon sources. Scaling production of PHAs that rely on these sources can affect land use, food prices, and environmental conditions depending on how the foods are grown and managed (Jiang et al., 2016). However, this food-based feedstocks challenge is also among the factors related to the current high costs associated with PHA production. Jiang et al.’s research looked at how to integrate PHA production with modern biorefineries to produce biofuels and bioplastics simultaneously. The authors advocate for more research on the topic, but initial estimates indicate that there is potential to offset the production cost of biofuels and reduce the overall production cost of PHAs using such an approach.
Another resource-related matter connected to PHA production in laboratories is that it involves a process of agitation-aeration in submerged fermentation, which requires large amounts of water, the use of solvents at different points in the process, and generates effluents that need to be treated (de Castro et al., 2022). Recycling of wastewater is a solution that we will also revisit in our blog on PHA production (Chen et al., 2020).
In their overview of PHAs, Pratt et al. (2019) acknowledge the wealth of literature that focuses on the use of organic feedstocks to increase PHA yield, but that there is a lack of research on resulting polymer quality and processing. The authors include a discussion by Gurieff and Lant (2007) that assessed the economics and carbon footprint of mixed culture PHA production (using an industrial wastewater as feedstock) with pure culture production. They found that mixed culture production was financially attractive in comparison to pure culture PHA production, and that the carbon footprints of both processes were similar and significantly lower than the production of high-density polyethylene (HDPE).
Conclusion
The advantages and challenges of PHAs can vary depending on the specific application and the formulation of the material. Despite the high costs of production, there is growing market potential for PHA polymers as promising replacements for the petroleum-based plastics.
PHAs also have great potential within the circular economy, wherein products are designed to be reused or recycled instead of becoming waste. The biodegradability of PHAs allows them to return to the ‘loop’ as feedstock for another process. As polymers are composted, they are mineralized to carbon dioxide and then reabsorbed during photosynthesis by the plants that can serve as feedstock for the next round of PHA production. This process closes the carbon cycle loop, demonstrating the value of PHAs within a circular economy (Adeleye et al., 2020; Braunegg et al., 2004).
As research and development continues, especially with regard to cost and scaling production, PHAs show promise of becoming an even more attractive alternative to conventional plastics within a fast-growing bioplastics industry.
We are a team of molecular biologists, geneticists, and bioinformaticians brought together by the desire to solve difficult problems with far-reaching implications.
Address
© 2025 All Rights Reserved. Website by Astrael



